Natural Products: Divine Inspiration for Chemistry
For synthetic organic chemistry to reach its full potential as an enabling science, especially in terms of its impact on biomedical research, we must develop the skills not only to prepare any molecule efficiently, on-scale, and in environmentally benign ways, but also to tailor it in any way imaginable so that we can confer upon it whatever new properties we might desire. Meeting this lofty objective requires complete command over chemoselectivity: the ability to differentiate and controllably functionalize any site within a given molecule.
Our research program uses the inspiration of Nature’s chemoselective syntheses of tens of thousands of natural products through highly controlled cyclization and functionalization chemistries to provide a sense of our ultimate goal and to identify critical challenges worthy of laboratory execution. Efforts to date have focused on oligomeric polyphenols, halogenated natural products, stereochemically-dense terpenes, and polycyclic alkaloids, and have afforded several new reagents, strategies, and tactics of broad applicability.
For instance, we have developed a broadly effective class of chemoselective halogenating reagents, of which Et2SBr•SbCl5Br (BDSB) is the flagship member and which is commercially available from Sigma–Aldrich. This and related materials are the first which can achieve halonium-induced polyene cyclizations for highly diverse terpenes, evidenced by the accomplishment of 6 total and formal total syntheses (including peysonnol A). They have also allowed the rapid synthesis of 8- and 9-membered bromoethers of the Laurencia class, as in laurefucin, via a unique biogenetic hypothesis where other bromonium sources were ineffective as well as accomplished positionally selective electrophilic aromatic substitutions that other halogen sources could not achieve. Some chiral variants of these materials have shown initial promise in achieving challenging asymmetric halogenations of isolated alkenes, work which builds upon our having achieved the first example of a highly enantioselective alkene dichlorination as part of a total synthesis of napyradiomycin A1.

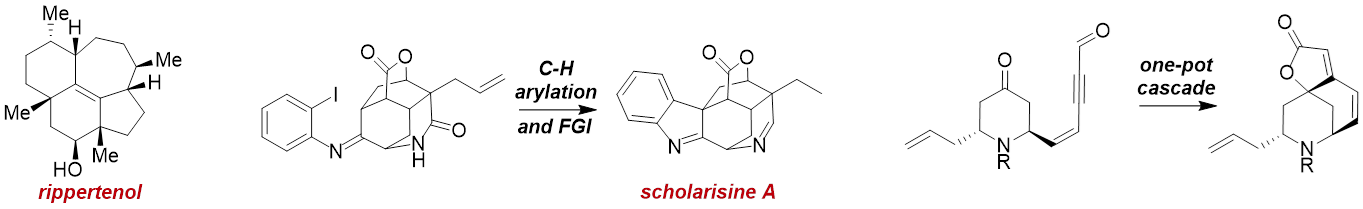
We have also developed a number of strategies and methods for rapid polycycle construction, evidenced by a recent total synthesis of the stereochemically rich, but largely non-functionalized terpene known as rippertenol. In work towards compact alkaloids such as scholarisine A and members of the norsecurinines, we have developed unique C-H functionalization chemistries and N-heterocyclic carbene-induced cascade bond constructions which can rapidly form multiple ring systems at once where previous efforts required many steps. As such, we hope these efforts can fuel further studies into their chemical biology with suitable material supplies now available.
We have also pioneered the first strategy, predicated on the identification of non-obvious starting materials and a number of chemoselective transformations and reaction cascades, for the controlled (and sometimes gram-scale) synthesis of diverse oligomeric natural product families, unlocking their members for further biological studies. These efforts include dozens of members of the resveratrol, rosmarinic acid, myrmicarin, and coccinellid families of natural products.

